Step-by-step explanation:
We are given a mixture of hydrochloric acid (HCl) and acetic acid
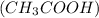 . The given mixture is a mixture of strong acid and a weak acid.
. The given mixture is a mixture of strong acid and a weak acid.
Strong acid is defined as the acid that can be get completely ionized or dissociated in an aqueous solution. Weak acid is defined as the acid which does not dissociate completely in an aqueous solution.
When a mixture of HCl and
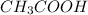 is titrated against NaOH (strong base), strong acid will get neutralized first because it is completely dissociated in the solution and can be easily neutralized.
is titrated against NaOH (strong base), strong acid will get neutralized first because it is completely dissociated in the solution and can be easily neutralized.