Answer: Thus for
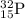 , no of electrons = no of protons = 15, number of neutrons = 17
, no of electrons = no of protons = 15, number of neutrons = 17
For
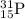 , no of electrons = no of protons = 15, number of neutrons = 16.
, no of electrons = no of protons = 15, number of neutrons = 16.
Step-by-step explanation:
Isotopes are elements which have same atomic number but different mass number.
General representation of an element is given as:
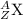
where,
Z represents Atomic number
A represents Mass number
X represents the symbol of an element
Thus the isotopes of phosphorous are represented as
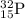 and
and
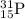
An atom is smallest unit of a matter or substance.It consist of three subatomic particles : electrons, protons, neutrons.
Mass number is defined as the sum of number of protons and neutrons that are present in an atom.
Mass number = Number of protons + Number of neutrons
Atomic number is defined as the number of protons or number of electrons that are present in an atom.
Atomic number = Number of electrons = Number of protons
Thus for
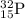 , no of electrons = no of protons = 15 and number of neutrons = 32-15= 17.
, no of electrons = no of protons = 15 and number of neutrons = 32-15= 17.
Thus for
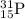 , no of electrons = no of protons = 15 and number of neutrons = 31-15= 16.
, no of electrons = no of protons = 15 and number of neutrons = 31-15= 16.