Oxidation reduction reaction or redox reactions are those in which there is change in oxidation number of any element
In general oxidation is defined as gain of oxygen or loss of electron or hydrogen by an atom. Reduction is defined as gain of electron or hydrogen or loss of oxygen by an atom.
Let us consider each reaction one by one
1)
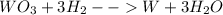
Here the oxidation number of W changes from +6 to 0 [reduction]
The oxidation number of H changes from 0 to +1 [oxidation]
Hence it is an oxidation reduction reaction
2)

Here there is no change in oxidation number of either element hence it is not oxidation reduction reaction
3)

Here there is no change in oxidation number of either element hence it is not oxidation reduction reaction
4)

Here there is no change in oxidation number of either element hence it is not oxidation reduction reaction