Let us consider each statement one by one
a) Catalysts are the substance which alter the reaction pathway where activation energy of reaction is less that the un catalyzed reaction. They are not used up in the reaction . the catalyst is restored in the reaction.
b) As mentioned above, the catalyst speed up reaction by lowering the activation energy
c) During a reaction the reactants are consumed up and products are formed. so there is a decrease in concentration of reactant.
Thus all the above statements are true.
D) the rate of decrease in concentration of reactants depends upon the coefficient of reactant in balanced chemical reaction.
For example
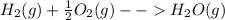
Here the rate of decrease in concentration of hydrogen will be double the rate of decrease in concentration of oxygen.