The acids react with base and the reaction is known as neutralization
One mole of hydronium ion reacts with one mole of hydroxide ion
Here
a) HI is a monoprotic acid : one mole of HI will give one mole of hydronium ion
b) CsOH is a monohydroxide base : one mole of CsOH will give one mole of hydroxide ion
So one mole of HI will react with one mole of CsOH
The moles of CsOH taken will be calculated from molarity and volume as
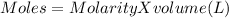
Thus moles of CsOH are
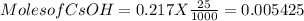
moles of HI required is 0.005425 mol
volume of HI required will be
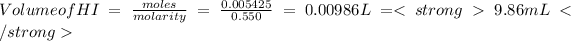
Volume of HI required is 9.86 mL