Given :
- Amount = 20 kg
- Heat energy absorbed = 237,000 J
- Temperature change = 15 °C
Formula applied :
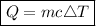
- Q = absorbed heat
- m = mass
- c = specific heat capacity
- ΔT = temperature change
Let's solve for c !
⇒ 237,000 = 20 × c × 15
⇒ c = 237,000 ÷ 300
⇒
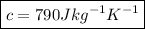
∴ The specific heat capacity of granite is 790 J kg⁻¹ K⁻¹.