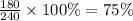Answer: B.) 25% unchanged and 75% stable
Explanation:
Now, to calculate the initial amount of radioactive isotope
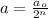
where,
a = amount of reactant left after n-half lives = 60 g
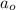 = Initial amount of the reactant = ?
= Initial amount of the reactant = ?
n = number of half lives= 2
Putting values in above equation, we get:
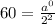
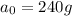
Thus percentage of sample unchanged=
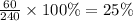
The percentage of sample decayed or has become stable=