Answer:
The mass of the platinum sample is greater than the mass of the lead sample (option C.)
Step-by-step explanation:
Density is a magnitude that allows you to measure the amount of mass in a given volume of a substance. Then, the expression for the calculation of density is the ratio between the mass of a body and the volume it occupies:
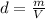
From this expression it can be deduced that the density is inversely proportional to the volume: the smaller the volume occupied by a given mass, the greater the density.
The density of lead is 11.3
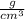 . Then it is possible to apply a rule of three as follows: if in 1 cm³ of lead there is 11.3 g of substance, in 2 cm³ how much mass is there?
. Then it is possible to apply a rule of three as follows: if in 1 cm³ of lead there is 11.3 g of substance, in 2 cm³ how much mass is there?
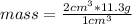
mass=22.6 g
The density of platinum is 21.5
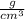 . Then it is possible to apply a rule of three as follows: if in 1 cm³ of platinum there is 21.5 g of substance, in 2 cm³ how much mass is there?
. Then it is possible to apply a rule of three as follows: if in 1 cm³ of platinum there is 21.5 g of substance, in 2 cm³ how much mass is there?
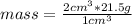
mass=43 g
Then, you can see that the mass of the platinum sample is greater than the mass of the lead sample (option C.)