Answer:
The correct answer is option b) O₂; 6.0 L C0₂
Step-by-step explanation:
The balanced reaction that occurs in this case is:
CS₂ (g) + 3 O₂(g)----> CO₂(g) + 2 SO₂(g)
The STP conditions refer to the standard temperature and pressure. Pressure values at 1 atmosphere and temperature at 0 ° C are used as reference values for gases. And in these conditions 1 mole of any gas occupies an approximate volume of 22.4 liters.
The rule of three or is a way of solving problems of proportionality between three known values and an unknown value, establishing a relationship of proportionality between all of them. That is, what is intended with it is to find the fourth term of a proportion knowing the other three. Remember that proportionality is a constant relationship or ratio between different magnitudes.
If the relationship between the magnitudes is direct, that is, when one magnitude increases, so does the other (or when one magnitude decreases, so does the other) , the direct rule of three must be applied. To solve a direct rule of three, the following formula must be followed:
a ⇒ b
c ⇒ x
So
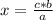
The limiting reagent is one that is consumed first in its entirety, determining the amount of product in the reaction. When the limiting reagent is finished, the chemical reaction will stop.
To determine the limiting reagent, it is possible to use the reaction stoichiometry of the reaction (that is, the relationship between the amount of reagents and products in a chemical reaction), and the amount of moles calculated as follows, taking into account that the reaction occurs under STP conditions and using the rule of three:
- if 1 mole of gas occupies 22.4 L, how many moles of CS2 occupy 11 L?
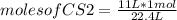
moles of CS2=0.49
- if 1 mole of gas occupies 22.4 L, how many moles of O2 occupy 18 L?
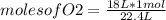
moles of O2= 0.80
Then, to determinate the limiting reagent, you can use a simple rule of three as follows: if by stoichiometry one mole of CS2 reacts with 3 moles of O2, how much moles of O2 will be needed if 0.49 moles of CS2 react?
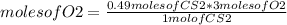
moles of O2=1.47
But 0.80 moles of O2 are available. Since you have less moles than you need to react with 0.49 moles of CS2, oxygen O2 will be the limiting reagent.
Then, it is possible to determine the amount of moles of CO2 produced by another rule of three: if by stoichiometry 3 moles of O2 produce 1 mole of CO2, how many moles of CO2 will be formed if 0.80 moles of O2 react?
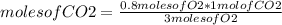
moles of CO2=0.27
Finally, taking into account the previously stated STP conditions and the amount of moles of CO2 formed, it is possible to determine the volume of CO2 that is formed by a rule of three: if 1 mole occupies 22.4 L, how much volume occupies 0.27 moles?
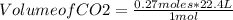
Volume of CO2=6.048 L≅ 6.0 L
Then, the correct answer is option b) O₂; 6.0 L C0₂