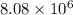Answer : The number of molecules inside the cell is,
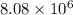
Solution :
First we have to calculate the volume of biomolecule cell.
Volume = Area of the base of the cell × length of the cell
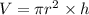
where,
V = volume of the biomolecule cell
r = radius of the cell =
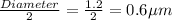
h = length of the cell =
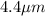
Now put all the given values in the above volume formula, we get
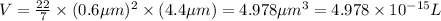
conversion :
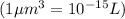
Now we have to calculate the moles of a biomolecule of the cell.
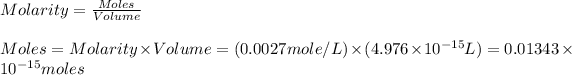
Now we have to calculate the number of molecules inside the cell.
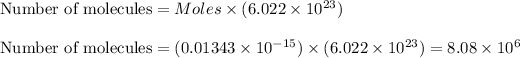
Therefore, the number of molecules inside the cell is,