Answer : Yes , solution of
 contains ions.
contains ions.
Step-by-step explanation:
The ionization reaction of sulfuric acid is given by the chemical equation, which follows:
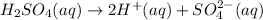
The sulfuric acid solution contains
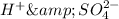 ions.
ions.
By stiochoimetery of the reaction;
In 2 mole of sulfuric acid solution there are 1 mole of [trex]H^+[/tex] ions and 1 mole of sulfate ions (
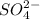 ).
).