Answer: [Ar]4s1
Explanation: Potassium
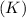 is an element with atomic number 19, thus it contains 19 electrons. It is a metal which readily loses its electrons to acquire stable configuration of Argon
is an element with atomic number 19, thus it contains 19 electrons. It is a metal which readily loses its electrons to acquire stable configuration of Argon
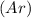 which has 18 electrons with completely filled subshells.
which has 18 electrons with completely filled subshells.
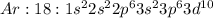
![K:19:[Ar]4s^1](https://img.qammunity.org/2020/formulas/chemistry/middle-school/skem78x49d2s56v3xoehmr4knc1c90zcap.png)