This is known that the number of moles of solute will remain the same even on dilution.
The relation between mole and molarity is
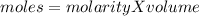
so the moles before and after dilution will be equated as:
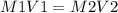
Where
M1=initial molarity = 15.9 M
V1= initial volume = ?
M2= Final molarity = 5 M
V2 = final volume = 1.80 L
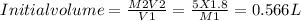
Thus we will take 0.566 L of concentrated nitric acid and will dilute it to 1.8 L.