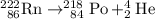
Y is Po-218.
Explanation
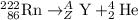
Here's the symbol of a particle in a nuclear reaction.
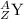 .
.
A stands for mass number. Z stands for atomic number. Both numbers shall conserve in a nuclear reaction.
- The mass number on the left hand side is 222.
- The two mass numbers on the right hand side add up to A + 4.
- 222 = A + 4.
- A = 218
So is the case for the atomic number. Try figure out the atomic number of Y using the same approach.
- The atomic number on the left hand side is 86.
- The two atomic number on the right hand side add up to __ + __.
- 86 = __ + __.
- Z = 84.
What element is Y? The atomic number of Y is 84. Refer to a periodic table. Element 84 corresponds to Po (polonium). Y is Polonium-218. The symbol of Y should be written as
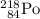 . Hence the equation:
. Hence the equation:
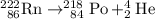 .
.