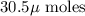Answer: The micro-moles of copper (II) fluoride is
Step-by-step explanation:
Molarity of a solution is defined as the number of moles of solute present in 1 liter of solution.
Mathematically,
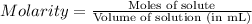
We are given:
Molarity of solution =
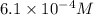
Moles of solute = ? moles
Volume of the solution = 50 mL = 0.05L (Conversion factor: 1L = 1000mL)
Putting values in above equation, we get:
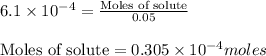
To convert it into micro-moles, we multiply it by
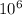
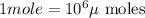
Converting
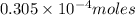 into micro-moles:
into micro-moles:
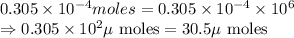
Hence, the micro-moles of copper (II) fluoride is