we can use the combined gas law equation
this shows the relationship between pressure, volume and temperature for a fixed mass of gas.
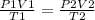
P1 is pressure, V1 is volume and T1 is temperature in Kelvin for the first instance
P2 is pressure, V2 is volume and T2 is temperature for the second instance.
Temperature is given in °C we have to convert it to Kelvin
T1 is - 89 °C + 273 = 362 K
T2 is - 25 °C + 273 = 298 K
substituting the given values in the equation
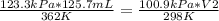
V2 = 126.4 mL
the final volume is 126.4 mL