Answer :
(a)
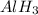
In this Lewis dot structure, there are 3 bond pair and 0 lone pair on the central atom. The total number of electron pair is 3. So, the hybridization of the molecule will be,
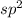 and the geometry will be, trigonal planar.
and the geometry will be, trigonal planar.
(b)
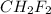
In this Lewis dot structure, there are 4 bond pair and 0 lone pair on the central atom. The total number of electron pair is 4. So, the hybridization of the molecule will be,
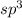 and the geometry will be, tetrahedral.
and the geometry will be, tetrahedral.
(c)
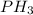
In this Lewis dot structure, there are 3 bond pair and 1 lone pair on the central atom. The total number of electron pair is 4. So, the hybridization of the molecule will be,
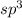 but the the fourth position will be occupied by one lone pair. Hence, the geometry will be, trigonal pyramidal not tetrahedral.
but the the fourth position will be occupied by one lone pair. Hence, the geometry will be, trigonal pyramidal not tetrahedral.
(d)
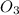
In this Lewis dot structure, there are 2 bond pair and 1 lone pair on the central atom. The total number of electron pair is 3. So, the hybridization of the molecule will be,
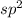 but the the third position will be occupied by one lone pair. Hence, the geometry will be, bent not linear.
but the the third position will be occupied by one lone pair. Hence, the geometry will be, bent not linear.
The image is also shown below.