Step-by-step explanation:
1) Boyle's Law: This law states that pressure is inversely related to the volume occupied by the gas at constant temperature and number of moles.
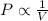 (At constant temperature and number of moles)
(At constant temperature and number of moles)
- When the size of the chamber is increased the volume occupied the gas will increase with which pressure exerted by the gas will decrease down.
- When we press the inflated balloon the pressure on the gas is increased with which volume of the occupied by the gas inside the balloon decreased.
2) Charles' Law: This law states that volume occupied by the gas is directly related to the temperature of the gas at constant pressure and number of moles.
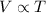 (At constant pressure and number of moles)
(At constant pressure and number of moles)
- The size of the balloon deceases because the in winters the temperature decreases with which volume of the gas present in the balloon also decreases.
- When the flexible closed container is heated the temperature of the gas inside the container increases with which the volume occupied by the gas in the container will increase resulting in expanding of container.
3) Avogadro's Law: This law states that volume occupied by the gas is directly related to the number of moles of the gas at constant pressure and temperature.
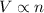 (At constant temperature and pressure)
(At constant temperature and pressure)
When we blow air into the balloon the umber of air particles increases with which the volume of the gas inside the balloon also increases resulting in increase in size of the balloon.