Answer:
Work done done by a system on its environment is W=40 Joules
Step-by-step explanation:
Given:
Ideal gas is maintained at a constant pressure
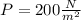
Change in Volume
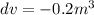
To find:
Work done by a system during an Isobaric Process
Solution:
In a Isobaric Process, constant pressure is maintained,
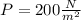
According to the formula,
work done
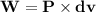
Substitute the values of pressure and change in volume in the above formula,
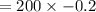
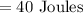
Result:
Work done done by a system W= -40 Joules