LiBr.
Step-by-step explanation
Note that the group number in this answer refers to the new IUPAC group number, which ranges from 1 to 18. Counts from the left. Start with the first two column (group 1 and 2), go on to the transition elements (Sc, Ti, etc. in group 3 through 12), and continue with the nonmetals (group 13 through 18).
Li is a group 1 metal. As a metal, it tends to form positive ions ("cations"). Metals in group 1 and 2 are main group metals. The charge on main group metal ions tends to be the same as the group number of the metal. Li is in group 1. The charge on an Li ion will be +1. Formula of the Li ion will be
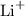 .
.
Br is a group 17 nonmetal. As a nonmetal, it tends to form negative ions ("anions"). The charge on nonmetal ions excepting for H tends to equal the group number of the nonmetal minus 18. Br is in group 17. The charge on a Br ion will be 17 - 18 = -1. Formula of the Br ion will be
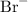
All the ions in an ionic compound carry charge. However, some of the ions like
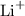 are positive. Others ions like
are positive. Others ions like
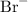 are negative. Charge on the two types of ions balance each other. As a result, the compound is overall neutral.
are negative. Charge on the two types of ions balance each other. As a result, the compound is overall neutral.
1 × (+1) + 1 × (-1) = 0. The positive charge on one
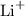 ion balances the negative charge on one
ion balances the negative charge on one
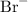 ion. The two ions would pair up at a 1:1 ratio.
ion. The two ions would pair up at a 1:1 ratio.
The empirical formula for an ionic compound shows all the ions in the compound. Positive ions are written in front of negative ions.
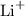 is positive and
is positive and
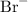 is negative. The formula shall also show the simplest ratio between the ions. For the compound between Li and Br, a 1:1 ratio will be the simplest. The "1" subscript in an empirical formula can be omitted. Hence the formula: LiBr.
is negative. The formula shall also show the simplest ratio between the ions. For the compound between Li and Br, a 1:1 ratio will be the simplest. The "1" subscript in an empirical formula can be omitted. Hence the formula: LiBr.