Answer: The correct answer is the oxidation state of at least two atoms should change.
Step-by-step explanation:
Redox reaction is defined as the reaction in which oxidation and reduction reaction takes place simultaneously.
Oxidation reaction is defined as the chemical reaction in which an atom looses its electrons. The oxidation number of the atom gets increased during this reaction.
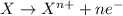
Reduction reaction is defined as the chemical reaction in which an atom gains electrons. The oxidation number of the atom gets reduced during this reaction.
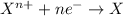
In a redox reaction, oxidation state of at least two atoms should change because here exchange of electrons takes place.
For Example: The reaction of copper with silver nitrate, the equation follows:
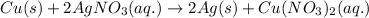
The half reactions for the above reaction are:
Oxidation half reaction:
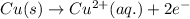
Reduction half reaction:
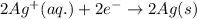
Oxidation state of copper and silver is getting changed to its respective ions.
Hence, the correct answer is the oxidation state of at least two atoms should change.