Answer:- 375 mL, last choice is correct.
Solution:- The problem is based on Boyle's law. From this law, At constant temperature the volume of the gas is inversely proportional to the pressure.
The equation used for this gas law is:
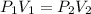
 is initial pressure,
is initial pressure,
 is final pressure.
is final pressure.
 is initial volume and
is initial volume and
 is final volume.
is final volume.
From given data, the initial pressure is 0.750 atm, initial volume is 300.0 mL.
Final pressure is 0.600 atm and we are asked to calculate the final volume.
Let's plug in the values in the equation:
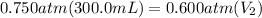
Let's rearrange this for final volume.
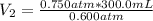
 = 375 mL
= 375 mL
So, the new volume of the gas is 375 mL, last choice is correct.