Answer:- 27.9 g of hydrogen.
Solution:- The given balanced equation is:
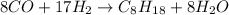
The problem asks to calculate the grams of hydrogen required to react completely with 6.50 moles of CO.
We will start with 6.50 moles of CO and multiply it by the mol ratio to get the moles of hydrogen. Looking at the balanced equation, there is 8:17 mol ratio between CO and
 .
.
In next step, the moles of hydrogen are converted to grams on multiplying the moles by molar mass.
Molar mass of
 is 2.02 g per mol. The set up is made using dimensional analysis and shown below:
is 2.02 g per mol. The set up is made using dimensional analysis and shown below:
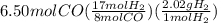
= 27.9 g

So, 27.9 grams of [tex]H_2] will react completely.