Answer:- New pressure is 0.942 atm.
Solution:- The volume of the glass bottle would remain constant here and the pressure will change with the temperature.
Pressure is directly proportional to the kelvin temperature. The equation used here is:
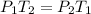
Where,
 and
and
 are initial and final temperatures,
are initial and final temperatures,
 and
and
 are initial and final pressures.
are initial and final pressures.
 = 20.3 + 273.15 = 293.45 K
= 20.3 + 273.15 = 293.45 K
 = -2.0 + 273.15 = 271.15 K
= -2.0 + 273.15 = 271.15 K
 = 1.02 atm
= 1.02 atm
 = ?
= ?
Let's plug in the values in the equation and solve it for final pressure.
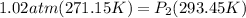
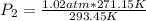
 = 0.942 atm
= 0.942 atm
So, the new pressure of the jar is 0.942 atm.