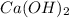Answer: The empirical formula of the compound is
Step-by-step explanation:
To find the empirical formula of the compound, we follow following steps:
- Step 1: Finding the number of moles of the given elements.
To calculate the number of moles, we use the formula:

Molar mass of calcium = 40 g/mol
Given mass of calcium = 13.5 g
Putting values in above equation, we get:
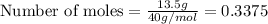
Molar mass of oxygen = 16 g/mol
Given mass of oxygen = 10.8 g
Putting values in above equation, we get:
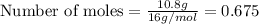
Molar mass of hydrogen = 1 g/mol
Given mass of hydrogen= 0.675 g
Putting values in above equation, we get:
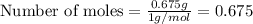
- Step 2: Now, to obtain the mole ratio, we divide the moles of each element by the smallest number of moles calculated.
For Ca =
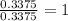
For H =
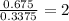
For O =
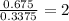
The ratio of Ca : O : H = 1 : 2 : 2
- Step 3: Now, the ratio of the elements is represented as the subscripts in the empirical formula.
The formula becomes:
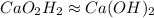
Hence, the empirical formula of the compound is