Answer: The correct statement is electrons are transferred from the lithium atom to the chlorine atom.
Step-by-step explanation:
Ionic bond is formed when there is complete transfer of electrons from one atom to another atom. The atom which donates electron is known as electropositive atom and the atom which accepts electron is known as electronegative atom.
Lithium is the 3rd element of the periodic table with electronic configuration
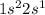
This atom can loose 1 electron and form
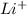 ion.
ion.
Chlorine is the 17th element of the periodic table with electronic configuration
![[Ne]3s^22p^5](https://img.qammunity.org/2020/formulas/biology/middle-school/nrguw6av1bhqb4oeax0gjg9zl6l72h372a.png)
This atom can gain 1 electron and form
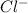 ion.
ion.
Hence, n electron is transferred from lithium to chlorine atom which results in the formation of ionic bond.
Thus, the correct statement is electrons are transferred from the lithium atom to the chlorine atom.