Explanations:- The balanced equation for the reaction is:
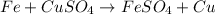
From above reaction, there is 1:1 mol ratio between Fe and Cu. It means 1 mol of Fe will produce 1 mol of Cu.
The atomic masses of Fe and Cu are different and for this reason 2.54 g of fe does not produce 2.54 g of Cu.
Below are the calculations that makes it more clear.
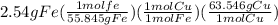
= 2.89 g Cu
So, calculations show that 2.54 g of Fe will produce 2.89 g of Cu. The difference is just because of the difference in their atomic masses.