Answer:- Synthesis.
Explanations:- Two species are reacting to make a newer one and so it is an example of a synthesis reaction.
If an equation is of the type,
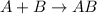 then it is called synthesis reaction.
then it is called synthesis reaction.
The given reaction in the question is,
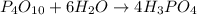
The above reaction looks exactly similar to the general equation we have written in the starting.
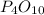 and water are reacting to form phosphoric acid and so it is a synthesis reaction.
and water are reacting to form phosphoric acid and so it is a synthesis reaction.