Answer : The mass of ethylene glycol is, 437.34 grams
Solution : Given,
Volume of ethylene glycol = 394 ml
Density of ethylene glycol = 1.11 g/ml (Standard value)
Formula used :

Now put all the given values in this formula, we get the mass of ethylene glycol.
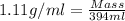
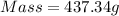
Therefore, the mass of ethylene glycol is, 437.34 grams