Answer: Option (d) is the correct answer.
Step-by-step explanation:
It is known that water boils at
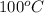 , that is, at this temperature liquid state of water starts to convert into steam or gaseous phase.
, that is, at this temperature liquid state of water starts to convert into steam or gaseous phase.
This means that, at
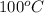 there are two phases present which are liquid and gaseous phase.
there are two phases present which are liquid and gaseous phase.
Also, the temperature remains constant unless and until the whole water changes from liquid to gaseous state.
Hence, we can conclude that in section D there are liquid and gaseous phase present.