Answer: The mole ratio of carbon and oxygen in the given chemical reaction will be 2 : 1
Step-by-step explanation:
Mole ratio is defined as the ratio of number of moles of the two substances taken into account or the ratio of their respective stoichiometric coefficients.
The given chemical equation follows:
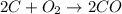
Moles of carbon atom = 2
Moles of oxygen gas = 1
Moles of carbon monoxide gas = 2
So, the mole ratio of carbon and oxygen = 2 : 1
Hence, the mole ratio of carbon and oxygen in the given chemical reaction will be 2 : 1