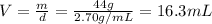Answer:
C) 16.3 ml
Step-by-step explanation:
Density is equal to the ratio between the mass of an object and its volume:
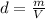
where
m is the mass
V is the volume
In our problem, we know:
- density of aluminium:
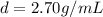
- mass of the aluminium foil:
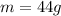
So we can re-arrange the equation above and use these data to find the volume of the piece of aluminium foil: