Step-by-step explanation:
An atom which has greater difference in the number of number of protons and neutrons will be unstable in nature. This is because then nuclear binding energy will not be strong enough to hold the neutrons and protons together.
For example,
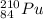 and
and
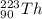 are both radioactive in nature due to large difference in the number of protons and neutrons.
are both radioactive in nature due to large difference in the number of protons and neutrons.
Whereas for
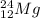 and
and
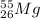 there is not much difference in the number of protons and neutrons.
there is not much difference in the number of protons and neutrons.
Thus, we can conclude that both Pu and Th elements tend to be radioactive in nature.