Recall this gas law:
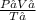 =
=
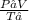
P₁ and P₂ are the initial and final pressures.
V₁ and V₂ are the initial and final volumes.
T₁ and T₂ are the initial and final temperatures.
Given values:
P₁ = 475kPa
V₁ = 4m³, V₂ = 6.5m³
T₁ = 290K, T₂ = 277K
Substitute the terms in the equation with the given values and solve for Pf:
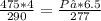
P₂ = 279.2kPa