Here we have to choose the correct statement on the effect of temperature on the motion of the molecules and atoms of a gas.
As the temperature increases the molecules and atoms move faster.
As per the kinetic theory of gas molecules and atoms the kinetic energy (K.E.) of the atom or molecules is related to temperature by the equation
K.E. =
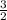 kT ( k = Boltzmann constant, T = temperature.
kT ( k = Boltzmann constant, T = temperature.
Thus as the temperature increases the K.E. increases thus the atom or molecules move faster.
With the decrease of temperature the movement of the atoms or molecules will be less and they will be near to each other.
The increment of temperature increase the K.E. thus the atoms or molecules move apart from each other.
With the decrease of temperature the movement of the atoms or molecules decreases.