The reaction between titanium ( IV) oxide and bromine trifluoride to give liquid bromine , and oxygen is:
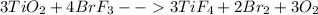
Thus from each three moles of titanium ( IV) oxide we will get three moles of oxygen molecule
The mass of oxygen obtained = 0.143g
The moles of oxygen obtained
=
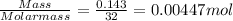
So moles of titanium ( IV) oxide required will be 0.00447mol
the molar mass of titanium ( IV) oxide is 79.87g/mol
mass of titanium ( IV) oxide used will be =
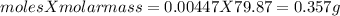
This mass of titanium ( IV) oxide is present in 2.367g of impure sample
the mass percent will be
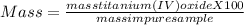
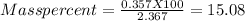
The percentage of titanium ( IV) oxide in impure sample is 15.08% (w/w)