Answer : The correct option is, (A) 2358
Step by step explanation :
Significant figure : It is defined as the minimum number of digit us needed to express a number in scientific notation.
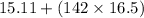
First we have to multiply the given digits as per rule.
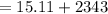
Now we will add both the digits, we get
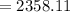
As per given expression, we conclude that the number of significant figure is 4. So, the answer will be, 2358
Hence, the correct option is, (A) 2358