Answer: 185.66 grams of Lithium chloride must decompose.
Step-by-step explanation:
To calculate the moles, we use the following equation:

Moles of Lithium:
Given mass of lithium = 30.3 grams
Molar mass of lithium = 6.91 g/mol
Putting values in above equation, we get:
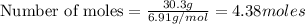
For the given chemical reaction, the equation follows:
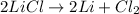
By Stoichiometry of the reaction:
2 moles of lithium metal are produced by 2 moles of lithium chloride
So, 4.38 moles of lithium metal are produced by =
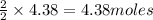 of lithium chloride.
of lithium chloride.
Now, to calculate the mass of lithium chloride, we use the moles equation:
Molar mass of lithium chloride = 42.39 g/mol
Putting values in above equation, we get:
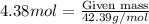
Mass of lithium chloride = 185.66 grams
Hence, Mass of lithium chloride decomposed is 185.66 grams.