Answer:
(D) quadruple
Step-by-step explanation:
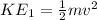 -----equation 1
-----equation 1
where;
KE₁ is the initial kinetic energy
m is the gas molecule mass
v is the velocity of gas molecules
⇒ When the velocity of gas molecules is doubled (2v)
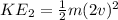
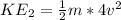
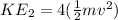 -------equation 2
-------equation 2
Recall; From equation 1;
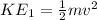
Substitute in KE₁ in equation 2
KE₂ = 4(KE₁)
Therefore, the kinetic energy will quadruple