Answer:
67.2 L is the volume of 3 moles of methane.
Step-by-step explanation:
Moles of methane gas = 3 mol
Given that 1 mol of any gas occupies 22.4 l of of volume under certain conditions of temperature and pressure.
Then volume occupied by 3 mol of meta he gas will be:
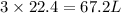
67.2 L is the volume of 3 moles of methane.