Empirical formula is the simplest ratio of components making up the compound. the molecular formula is the actual ratio of components making up the compound.
the empirical formula is CH₂. We can find the mass of CH₂ one empirical unit and have to then find the number of empirical units in the molecular formula.
Mass of one empirical unit - CH₂ - 12 g/mol x 1 + 1 g/mol x 2 = 12 = 14 g
Molar mass of the compound is - 252 .5 g/mol
number of empirical units = molar mass / mass of empirical unit
=
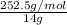
= 18 units
Therefore molecular formula is - 18 times the empirical formula
molecular formula - CH₂ x 18 = C₁₈H₃₆
molecular formula is C₁₈H₃₆