As given, it is an exothermic reaction, there is release of energy, so we will write the energy on product side.
The balanced equation of reaction of ethene with water will be:
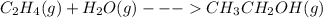
Now as we have to write a thermochemical equation we will write it as
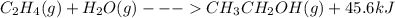
So when we have to write a balanced equation:
a) we should write all the compounds present in reactant side followed by product side
b) we balance all the elements on both the side
c) based on type of reaction we put energy on reactant / product side