Answer: The name of the
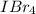 is Iodine tetrabromide and it is a covalent compound.
is Iodine tetrabromide and it is a covalent compound.
Step-by-step explanation:
Inter-halogen compounds are compounds which composed of two different halogen atoms. For example ;
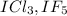 etc.
etc.
The naming is done by first writing the central atom symbol's name followed by the prefix corresponding to the number of atoms of another halogen atom like: Mono for 1, di ,for 2 tri for 3, tetra for 4 , penta for 5 etc. After this name of the another halogen is written with suffix 'ide' in the end.
The name of the
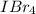 is Iodine tetra bromide.
is Iodine tetra bromide.
In halogen compounds, the difference in electronegativities is not so large due to which they form covalent compounds by the means of sharing the electrons. Hence the Iodine tetrabromide covalent compound.