Answer:The empirical formula of the compound will have a boron to hydrogen ratio of 1:3.
Step-by-step explanation
Percentage of Boron = 78.14 %
Percentage of Hydrogen = 21.86 %
Suppose in 100 gram of medicine
Mass of boron in 100 grams of medicine = 78.18 g
Mass of the hydrogen in 100 grams of medicine = 21.86 g
Moles of boron:
=
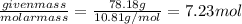
Moles of hydrogen:
=
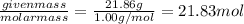
For the ratio of boron and hydrogen divide the number moles of respective elements with smallest number of moles calculated.
Here smallest numeric value moles are of boron which is 7.23 moles
For boron =
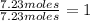
For hydrogen =
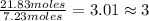
So ,the ratio of Boron to Hydrogen ; 1:3
The empirical formula of the compound will be :
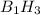
The empirical formula of the compound will have a boron to hydrogen ratio of 1:3.