Step-by-step explanation:
Enthalpy change is defined as in a chemical reaction the amount of energy absorbed or evolved in the form of heat at constant pressure.
For the given reaction,
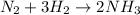 calculate the enthalpy change as follows.
calculate the enthalpy change as follows.
 = Bond energy of reactants - Bond energy of products
= Bond energy of reactants - Bond energy of products
= {[(2 × 942) + (3 × 432)] - [6 × 386]} KJ/mol
= {[1884 + 1296] - 2316} KJ/mol
= {3180 -2316} KJ/mol
= 864 KJ/mol
Thus, we can conclude that the enthalpy change for the given reaction is 864 KJ/mol.