Answer: The reactant ratio in the given chemical equation will be:

Step-by-step explanation:
Mole ratio is defined as the ratio of the amount of moles of two substances that are participating in a chemical reaction.
In the given chemical equation:

The reactants are Fe and
 and the product is
and the product is
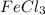
The mole ratio is basically the stoichiometric ratio of the chemical compounds taking part in a chemical reaction.
The mole ratio of reactants is stoichiometric ratio of Fe and
 , which is:
, which is:

Hence, the reactant ratio in the given chemical equation will be:
