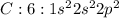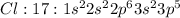Answer: False
Step-by-step explanation: Carbon is the central atom which has valency of 4 and can form 4 bonds to complete its octet. Each chlorine has valency of 1 and can form only one bond to complete its octet. Thus carbon can bond with four chlorine atoms through sharing of electrons to form four single bonds. Thus it cannot contain a double bond.