Answer: The substance which is slippery and conducts electricity in water is base.
The substance which continuously made bubbles of hydrogen gas when Jake dropped magnesium into an aqueous solution of the substance is acid.
Step-by-step explanation:
1. An acid is defined as a substance which dissociates to give
 ions when dissolved in water.
ions when dissolved in water.
They conduct electricity when dissolved in water due to the presence of ions.
Acids are sour in taste.
Acids react with metals to give hydrogen gas.
When magnesium is treated with a strong acid such as
 ,magnesium being more reactive than hydrogen displaces hydrogen from its salt solution and thus produce magnesium chloride and hydrogen gas.
,magnesium being more reactive than hydrogen displaces hydrogen from its salt solution and thus produce magnesium chloride and hydrogen gas.
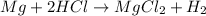
2. A base is defined as a substance which dissociates to give
 when dissolved in water.
when dissolved in water.
They conduct electricity when dissolved in water due to the presence of ions.
Bases are slippery in nature.
Thus we can conclude that the substance which is slippery and conducts electricity in water is base and the substance which continuously made bubbles of hydrogen gas when Jake dropped magnesium into an aqueous solution of the substance is acid.