Answer: The correct answer is Option B.
Step-by-step explanation:
For a reaction to be spontaneous, the Gibbs' Free Energy must be negative.
Equation for Gibbs Free energy is given by:
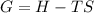
Where, G = Gibbs free energy
H = Enthalpy of the reaction
T = Temperature of the system
S = Entropy of the system
Sign convention for all the quantities:
For H: It is negative for exothermic reactions and positive for endothermic reactions
For S: It is positive when there is increase in entropy and it is negative when there is decrease in entropy.
If we want G to be negative, the conditions necessary are:
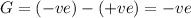
The product of temperature and entropy must be positive and that will be positive when there is increase in entropy at any temperature.
Hence, the correct answer is Option B.