Explanation :
Corrosion is the process of conversion of metal into its oxides, hydroxides and sulfides.
Rusting of iron is the process of corrosion in metals. Iron objects react with oxygen in the air and turned reddish. This reddish covering is Rust.
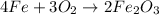
where,
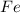 is iron
is iron
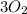 is oxygen present in atmosphere
is oxygen present in atmosphere
and
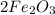 is rust or Ferric oxide.
is rust or Ferric oxide.
In the given equation,
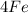 and
and
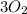 both are reactants and
both are reactants and
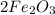 is a product.
is a product.